Salt Impacts & Our Environment
Excessive salts introduced to the environment can make their way from the ground surface into our freshwater systems including rivers, lakes, streams, and groundwater, where they can negatively impact aquatic plants and animals and potentially affect human health.

SURFACE WATERS | GROUNDWATER | PROTECTING OUR WATER | RESOURCES

How Salt Changes Water Chemistry
Chemically, salts are made of two parts, a positive ion (known as a cation) and a negative ion (known as an anion). Like batteries, these opposite charges are attracted to each other to form a chemical bond. The most common salt we are familiar with, and use daily, is sodium chloride (NaCl). When salt dissolves in water it breaks up into a positive, sodium ion (Na+) and a negative, chloride ion (Cl-). In small amounts, these ions are not problematic, but when there are a lot of these ions, they can be.
Sodium and chloride ions behave differently when salt is washed off hardscape surfaces, like roadways, walkways, and parking lots, into the soil.
Sodium ions are sometimes called “bullies” because they kick other ions off soil particles. Learn more about how salt changes soil chemistry here. When soil is packed with sodium ions from the over-use of salt, the excess sodium ions make their way into groundwater.
While sodium ions are “bullies” who cling to soil particles, chloride ions move freely through the soil into groundwater. Because they don’t react with soil, chloride ions can remain in groundwater for many years, or even decades. Once salt is added to water, the primary method to “fixing” the problem is allowing freshwater to replace the saltwater.

In this cartoon we see an example of what happens when salt interacts with soil and groundwater.
Panel 1: Salt (NaCl) breaks into sodium (Na+) and chloride (Cl-) ions in the groundwater surrounding soil particles. The soil particle has a mix of ions stuck to the surface including sodium (Na+), calcium (Ca2+), magnesium (Mg2+), and potassium (K+).
Panel 2: The sodium ions kick off calcium and magnesium ions from the surface of the soil particle. The chloride ions stay separate and move through the groundwater.
Panel 3: The calcium and magnesium ions move out into the groundwater while the sodium sticks to the soil particle.
Panel 4: The sodium ions are now the majority on the soil particle. The chloride ions remain in the groundwater.

Fresh groundwater naturally has minerals including sodium, magnesium, calcium, iron, manganese, chloride, and more. But when there is an excess of minerals, like salt, added to the groundwater, the water chemistry can become imbalanced. Too much sodium can cause more magnesium and calcium to release from soil and increase water hardness. Too much chloride can change water pH making the water more corrosive. Very hard water can quickly scale and clog household plumbing, while too much chloride can corrode and potentially pull harmful metals, like lead, out of the plumbing.
As salt over-use increases, we risk turning our freshwater saline. Scientists refer to the increasing salt concentrations of freshwater ecosystems as freshwater salinization.(1) Humans and many other organisms, rely on freshwater for survival. It’s important that we reduce our salt footprint today to protect our freshwater resources for future generations.
SURFACE WATERS
Stormwater
Stormwater is rain or snowmelt that runs off the landscape. In the natural environment, this water is absorbed by plants and soil as part of the water cycle. However, impervious (impenetrable) surfaces in developed areas such as rooftops, paved streets, highways, and parking lots, block this natural absorption from occurring. Stormwater will pick up and transport contaminants including salt, carrying them to the local streams, rivers, lakes, or even to Long Island Sound. Because of all the things that stormwater can pick up as it flows, it can be a significant source of pollution to the environment.

Salt can make its way into stormwater in a variety of ways including washing off treated surfaces, releases from improperly stored or uncovered salt piles in parking lots or at public works, or broken bags of salt left outside of stores.
Salty stormwater can have many consequences on the environment and on infrastructure. Salty stormwater that flows into fresh watercourses can change the water chemistry and impact aquatic plants and animals. It can also be corrosive to infrastructure like concrete storm drains and metal drainage pipes causing premature failures in the drainage system that can result in localized flooding, pollution of groundwater, or roadway damages.
To reduce potential impacts from stormwater pollution, DEEP’s Stormwater Program manages permits for municipalities and industrial and commercial businesses that maintain stormwater drainage systems. For more information on the stormwater management permits, go to Stormwater Management.
To learn more about how you can minimize releases of salt to stormwater, visit What Can I Do?
Surface Water Monitoring
DEEP staff in the Water Monitoring Program regularly monitor freshwater watercourses, such as streams and rivers, throughout Connecticut as part of the requirements of the federal Clean Water Act. Just like when your doctor takes your vital signs and conducts regular bloodwork to assess your health, DEEP staff collect data from watercourses to assess their health, or water quality conditions. These conditions include the watercourse’s chemical, physical, and biological properties, such as pH, temperature, flow rates, and the number and types of organisms living in the water. Just like it’s normal for our health vitals to change over time, it’s natural that some of these water quality conditions change over time. However, one of the functions of the monitoring program is to observe changes in conditions that may not be natural and may indicate pollution or impacts caused by human activities that have impaired the watercourse.
Healthy waters create a healthy ecosystem for plants and animals that live in that water (aquatic life) to thrive. Impaired waters are not able to sustainably support plants and animals. Impaired waters are identified based on specific values, or criteria, that are set by the State. The State sets criteria to protect aquatic life based on extensive scientific research. The goal for any watercourse is to stay below these defined criteria to maintain a healthy ecosystem. When these criteria are exceeded, there are negative consequences to aquatic organisms.
Two of the various quality measurements collected by DEEP staff include chloride and conductivity. Conductivity is a measure of the ability of water to pass an electrical current. Dissolved salts and other inorganic substances conduct electrical current. So, the higher the conductivity, the higher the concentrations of salts. Conductivity is used as a screening tool to check the “saltiness” of the water. DEEP staff collect samples for chloride analysis to confirm the true salt concentration.
Of these two water quality measurements, the State has set the acute (kills aquatic life quickly) criteria for chloride at 860 mg/L and the chronic (kills aquatic life slowly) criteria at 230 mg/L.
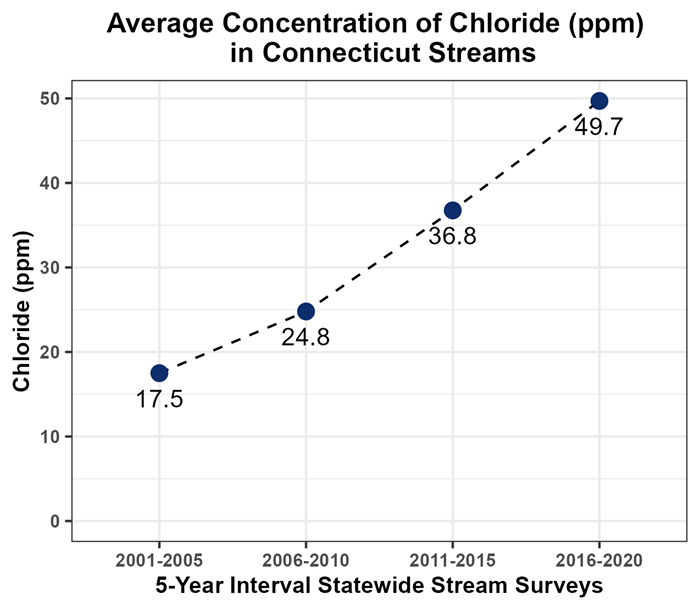
Since 2001, water quality data collected by DEEP have shown a concerning trend of increasing chlorides in Connecticut streams. DEEP staff completed four statewide surveys, each over a 5-year period from 2001 to 2020, to provide an estimate of stream water quality conditions across the entire State of Connecticut. As illustrated in the graph below, average Statewide chloride concentrations have more than tripled from 14.2 mg/L (2001-2005) to 49.7 mg/L (2016-2020). While the observed concentrations were typically less than the 230 mg/L chronic criteria, emerging scientific research suggests that even small increases of chloride can negatively affect stream ecosystems.(2,3) If we do not reduce the amount of salt introduced to the environment, it is possible chloride concentrations in Connecticut streams will reach the chronic criteria sooner than later.
The DEEP River and Stream Water Quality Monitoring program continues to collect conductivity, chloride, and biological data in Connecticut streams to assess impacts on aquatic life.
The United States Geological Survey (USGS) and other New England states, such as New Hampshire, have documented similar increasing chloride trends in their freshwater watercourses. In the early 2000s, the state of New Hampshire documented exceedances of the EPA acute and chronic criteria in four New Hampshire watersheds.( 4-7) The results of the study provided the necessary information for the State of New Hampshire Department of Environmental Services to develop an action plan to reduce the over-application of salt including establishing the original Green Snow Pro Program.
More information on Water Quality Standards, Water Monitoring Programs, Watershed Management, and more can be found starting on DEEP’s Integrated Water Quality report to Congress webpage.
GROUNDWATERWhen excess salt is introduced to soil and surface waters it can make its way down into the groundwater (infiltrates) where it can linger for years, even decades.
Aquifer Protection
An aquifer is any underground body of rock and/or sediment that allows for withdrawal of usable amounts of water. Groundwater can move through this rock or sediment and can be used as a source of drinking water.

More than 60% of Connecticut residents use groundwater for drinking water: more reason to take the protection of the groundwaters of the State very seriously! The DEEP Aquifer Protection Area Program (APA Program) protects groundwater that serves as a source to major public water supplies throughout Connecticut to ensure a plentiful supply of public drinking water is available for present and future generations.
If excess salt is introduced to any aquifer it could potentially contaminate sources of drinking water for many Connecticut residents. Once salt is added to water, there is no easy way to remove it other than through a reverse osmosis purification process which can be financially and practically challenging.
Regulations for proper salt storage have been established to minimize the risk of salt impacts to aquifers used for public drinking water. The APA Program depends on collaborative relationships with Connecticut municipalities and water companies to uphold these regulations to minimize the risk of contamination to these sensitive sources of public drinking water.
Watch our YouTube video to learn more about what an aquifer is.
To see if you live in an Aquifer Protection Area, check our Aquifer Protection Interactive Map.
Well Water
According to estimates provided by the CT Department of Public Health (DPH), approximately 23% of Connecticut residents are served by private water supply wells. Homeowners who own private wells are responsible for maintaining their own well and periodically checking the quality of their well water. The DPH Private Wells Program has many resources for homeowners including lists of State Certified Laboratories that will analyze residential water samples, tests to request, and more.
Like the criteria mentioned above for surface water, the DPH sets criteria for drinking water including Maximum Contaminant Levels (MCLs), Drinking Water Action Levels (DWALs), or guidance levels. These criteria are generally related to health risk. Connecticut has a set MCL for chloride of 250 mg/L and a guidance level for sodium at 100 mg/L.
Often homeowners report a salty taste to their water or excessive corrosion of household plumbing and appliances when they first realize they have elevated salt concentrations in their well water. Unfortunately, once salt has infiltrated the groundwater that feeds into a private well it is not easy to remove and can take years to resolve.
While DPH sets criteria for drinking water to protect human health, under Connecticut law (CGS 22a-471), DEEP’s Remediation Division is charged with protecting the groundwaters of the state used for drinking and domestic purposes from pollution. When drinking water is polluted with substances like salt, the Remediation Division works to identify potential responsible parties. Once identified, the Remediation Division notifies the potential responsible party(ies) that they must conduct a thorough investigation to assess and design solutions to provide the impacted property with a short-term (temporary) and a long-term (permanent) source of drinking water. Together, the Remediation Division and the identified party(ies) will work towards a resolution.
If you suspect you may have elevated salt concentrations in your well water and wish to have DEEP’s Remediation staff investigate, please visit our Road Salt Investigation page for details on how to file a complaint.
Reducing the amount of salt introduced to the environment may minimize the chances of polluting private wells and reduce the need for time-intensive and costly investigations.
PROTECTING OUR WATER
Dilution is part of the solution to salt pollution. In wet years, the rain and snow melt can help flush salt through soil into groundwater, which is beneficial to soil, but can potentially increase the salinity (the amount of dissolved salt) of fresh water sources and wildlife habitats. The only ways for fresh surface water and groundwaters to recover from salt pollution is to allow natural dilution to occur and to reduce the amount of salt added to the environment.
As climate change leads to more extreme weather, including droughts paired with more intense winter weather that could require more salt applications, there is greater risk of salt build-up in groundwater. Increased salt in groundwater could have ripple effects in the freshwater ecosystems that we rely on.
While dilution is part of the solution, it is out of our control; whereas reduction is within our control. Reducing the amount of salt we use can help reduce the negative impacts and benefit our freshwater ecosystems. We can make the difference.
Although many studies have investigated the impact of road salts on surface water and groundwater, it continues to be an ongoing area of research. There is more to learn about the impacts to the numerous species and various ecosystems that are impacted by salts. However, we do know that when ecosystems are degraded and biodiversity is lost due to salt impacts, the benefits we receive from those ecosystems can be lost. Therefore, if we reduce our salt use as a society, less salt will enter our environment and the known negative impacts to soils and plants will be reduced.
RESOURCES
1. Kaushal, S.S., Likens, G.E., Pace, M.L., Utz, R.M., Haq, S., Gorman, J., Grese, M. 2018. Freshwater salinization syndrome on a continental scale. PNAS, 115, E574-E583. https://doi.org/10.1073/pnas.1711234115
2. Hintz, W.D., Arnott, S.E., Symons, C.C., Weyhenmeyer, G.S. 2022. Current water quality guidelines across North America and Europe do not protect lakes from salinization. PNAS, 119. https://doi.org/10.1073/pnas.2115033119
3. Hintz, W.D. A review of the species, community, and ecosystem impacts of road salt salinisation in fresh waters. 2019. Freshwater Biology, 64, 1081-1097. https://doi.org/10.1111/fwb.13286
4. Harte, P.T., Trowbridge, P.R. 2010. Mapping of road-salt-contaminated groundwater discharge and estimation of chloride load to a small stream in southern New Hampshire, USA. Hydrol. Process. 24, 2349-2368. https://doi.org/10.1002/hyp.7645
5. Trowbridge, P.R., Kahl, J.S., Sassan, D.A., Heath, D.L., Walsh, E.M. 2010. Relating road salt to exceedances of the water quality standard for chloride in New Hampshire streams. Environ. Sci. Technol. 44, 4903-4909. https://doi.org/10.1021/es100325j
6. New Hampshire Department of Environmental Services. 2008. Total Daily Maximum Load (TMDL) Study for Waterbodies in the Vicinity of the I-93 Corridor from Massachusetts to Manchester, NH: Beaver Brook in Derry and Londonderry, NH
7. New Hampshire Department of Environmental Services. 2008. Total Maximum Daily Load (TMDL) Study for Waterbodies in the Vicinity of the I-93 Corridor from Massachusetts to Manchester, NH: Policy-Porcupine Brook in Salem and Windham, NH

FAQs | StoryMap: More Than Just a Grain | What Can I Do? | Resources

