Hepatitis A Surveillance in Connecticut 2015 - 2019
Authors: A. Bogacki, MPH, CHES, P. Gacek, MPH, CPH, Q. Phan, MPH, J, Sun, MD, PhD, J. Mullins, DVM, MPH
Hepatitis A infection is a highly contagious acute infection of the liver caused by the hepatitis A virus (HAV). The virus is primarily spread from person to person by the fecal-oral route, and household or sexual contacts of cases are at risk of becoming infected. Common signs and symptoms include fatigue, fever, malaise, diarrhea, loss of appetite, nausea, abdominal pain, dark urine, and jaundice. Some people do not have symptoms, others can experience a mild illness and recover within a couple of weeks, while still others develop more severe symptoms and may take months to fully recover (1).
The Connecticut Department of Public Health (CT DPH) uses the current National Surveillance Case Definition to classify hepatitis A cases (2). The clinical criteria used to define hepatitis A cases include an acute illness with a discrete onset of any sign or symptom consistent with acute viral hepatitis (e.g., fever, headache, malaise, anorexia, nausea, vomiting, diarrhea, abdominal pain, or dark urine) AND (a) jaundice or elevated total bilirubin levels ≥ 3.0 mg/dl, OR (b) elevated serum alanine aminotransferase (ALT) levels >200 IU/L, AND (c) the absence of a more likely diagnosis. The laboratory criteria for diagnosis includes a positive immunoglobulin M (IgM) antibody to hepatitis A virus (anti-HAV), OR a positive nucleic acid amplification test (NAAT; such as polymerase chain reaction [PCR] or genotyping) for hepatitis A virus RNA. A confirmed case is classified as a case that meets the clinical criteria and is IgM anti-HAV positive, OR a case that has hepatitis A virus RNA detected by NAAT (such as PCR or genotyping), OR a case that meets the clinical criteria and occurs in a person who had contact (e.g., household or sexual) with a laboratory-confirmed hepatitis A case 15-50 days prior to onset of symptoms, AND not otherwise ruled out by negative IgM anti-HAV or negative hepatitis A virus NAAT testing performed by a public health laboratory. Laboratory reports that do not result in a confirmed case are classified as non-cases.
In Connecticut, positive Immunoglobulin M antibodies to HAV (IgM anti-HAV) results are a laboratory reportable finding and HAV infection is a provider reportable disease. When a positive test result is received by CT DPH, follow-up is conducted with provider offices and infection control practitioners. If clinical findings result in a confirmed case, a telephone interview of the case patient is conducted by CT DPH staff using a standardized questionnaire. During the interview, the case is asked about symptom onset, specific symptoms experienced, occupation, food exposures, travel, and other questions important to identifying potential exposures. In addition, close contacts of the patient are encouraged to receive post exposure prophylaxis within 14 days of exposure to the case while infectious.
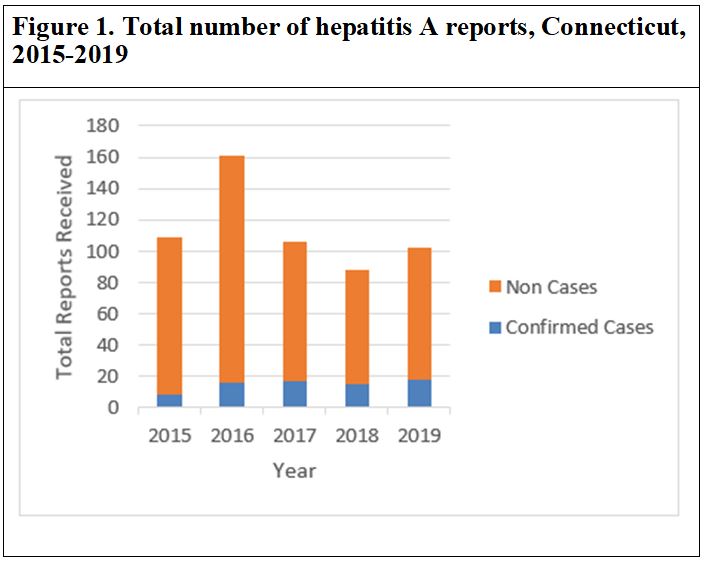
During 2015-2019, CT DPH received and conducted follow-up for 566 positive anti-HAV IgM reports, of which 74 (13%) were classified as confirmed cases and 492 (87%) were classified as non-cases (Figure 1). Further analyses were conducted on confirmed cases.
Statewide annual incidence rates ranged from a low of 0.25 cases per 100,000 population in 2015 to a high of 0.50 cases per 100,000 population in 2019. Compared to 2011 when the last published incidence showed a rate of 0.46 cases per 100,000 population, the cumulative average incidence rate for 2015-2019 was 0.41 per 100,000 population (3,4).
Cases ranged from 2 to 87 years of age with a median age of 48 years, with the highest proportion reported in those aged 50-59 years (24%); 48 (65%) were male. Race information was collected and included 52 (70%) White, 3 (4%) Asian, 1 (1%) Black or African American, 3 (4%) Other, and 15 (20%) unknown. Ethnicity was also collected in 73 (99%) cases and 13 (17%) identified as Hispanic/Latino. No cases reported having received hepatitis A vaccine.
Symptoms reported included 47 (64%) loss of appetite, 43 (58%) malaise, 43 (58%) dark urine, 42 (57%) jaundice, 39 (53%) nausea, 36 (49%) fever, 33 (45%) abdominal pain, 29 (39%) diarrhea, 24 (32%) vomiting, and 19 (26%) headache. Cases could have had more than one symptom. Additional symptoms such as bloating, chills, fatigue, weakness, and body aches were also reported. There were 43 (58%) hospitalizations; no deaths were reported.
Of the 74 cases, 26 (35%) reported international travel within 3 months prior to symptom onset, 19 (26%) consumed raw shellfish, 3 (4%) had contact with a person who traveled internationally, 3 (4%) used illicit injectable drugs, 3 (4%) were men having sex with men (MSM), and 1 (1%) used non-injectable illicit drugs. There were no reports of known contact with someone with infectious hepatitis A. There were 9 (12%) cases that reported working in “high-risk” occupations: 4 (6%) healthcare employees with direct patient care, and 2 (3%) as an employee or an attendee of a daycare center, nursery, or preschool.
Editorial
In the United States, hepatitis A rates decreased by more than 95% from 1996, when the vaccine was introduced, through 2011 (5). Nationally, cases started to increase in 2016, when large person-to-person outbreaks began to occur (1). Over time, the CDC has changed the national surveillance case definition. Results of liver enzyme tests, clinical findings, and other key factors used to classify a case were modified. In Connecticut, during 2015-2019, 87% of positive anti HAV-IgM tests resulted in a non-case after follow-up was conducted. CT DPH investigations determined more than 50% of non-cases were screened for HAV as part of routine blood work.
Certain groups of people are at higher risk for HAV infection. People who travel internationally and are unvaccinated are at a greater risk of getting HAV infection. In Connecticut, international travel was the predominant risk factor among cases. The CDC encourages all international travelers to get vaccinated to reduce their risk of contracting the disease (6). Other groups who are at increased risk for HAV infection are MSM, people who use illicit injection or non-injection drugs, people with occupational risk for exposure, and people experiencing homelessness. Healthcare workers and food workers who develop infection pose a greater risk to infect others and can cause outbreaks if proper control measures are not put in place.
Providers are encouraged to screen for HAV infection and test for HAV among patients at increased risk including having recent international travel, as well as those who show signs or symptoms. If providers have any questions or concerns about a positive hepatitis A patient, they are encouraged to call the Epidemiology and Emerging Infections Program at 860-509-7994.
The best way to prevent hepatitis A is to get vaccinated. Hand washing and good hygiene are essential to helping stop the spread of this disease (1). Acute cases require immediate follow-up to identify close contacts and reduce the potential of further transmission of disease
References1. Centers for Disease Control and Prevention. (2020, July 28). Hepatitis A Q&As for Health Professionals. Centers for Disease Control and Prevention. Retrieved June 9, 2021, from https://www.cdc.gov/hepatitis/hav/havfaq.html.
2. Centers for Disease Control and Prevention. (2021, April 16). Hepatitis A, acute| CDC. Centers for Disease Control and Prevention. Retrieved June 9, 2021, from https://ndc.services.cdc.gov/conditions/hepatitis-a-acute/.
3. Connecticut Department of Public Health. (n.d.). Annual state county population with demographics. CT.gov. Retrieved May 26, 2021, from https://portal.ct.gov/dph/Health-Information-Systems--Reporting/Population/Population-Statistics.
4. Connecticut Department of Public Health. The Changing Epidemiology of Hepatitis A Virus Infection (HAV) in Connecticut, 2006-2011. Connecticut Epidemiologist, 32,(5)
5. Centers for Disease Control and Prevention. (2021, August 18). Pinkbook: Hepatitis A. Centers for Disease Control and Prevention. Retrieved September 4, 2021, from https://www.cdc.gov/vaccines/pubs/pinkbook/hepa.html.
6. Centers for Disease Control and Prevention. (2021, October 15). Hepatitis A vaccine information statement. Centers for Disease Control and Prevention. Retrieved September 14, 2021, from https://www.cdc.gov/vaccines/hcp/vis/vis-statements/hep-a.html.
This page last updated 12/06/2021.

