| CTR Home | Data & Statistics | Research | Resources for Registrars | Reporting Requirements | Other Resources | Contact CTR|
CTR is a central resource for registrars working in all CT hospitals and healthcare facilities to ensure timely and accurate reporting on all cancer cases diagnosed within the state.
Resources
-
Lists The following lists detail specific cancer reporting requirements:
NAACCR Standards and Data Dictionary
NAACCR Required Data Items (2018-2019)
Change log - Solid Tumor Rules - December 2020 Revision
Effective Dates for Cancer Registry Reference Manuals
A Cancer Registrar's Guide to Collecting Industry and Occupation
NCI Dictionary of Cancer Terms Widget
Ill-defined and Unknown Primaries
Connecticut Historical Staging Manual (CANCER PROGNOSIS MANUAL)
-
Abstracts To assist registrars in preparing abstracts, NCRA’s Education Committee provides informational abstracts:
-
NAACCR Webinar Series Monthly series of 3-hour long webinars:
10/04/2018 Collecting Cancer Data: Lung
11/01/2018 Collecting Cancer Data: Pharynx
12/06/2018 Collecting Cancer Data: Breast
01/10/2019 Collecting Cancer Data: Testis
02/07/2019 Collecting Cancer Data: Colon
04/04/2019 Collecting Cancer Data: Hematopoietic and Lymphoid Neoplasms
05/02/2019 Collecting Cancer Data: Neuroendocrine Tumors
06/06/2019 Collecting Cancer Data: Ovary
02/06/2020 SDDI's: An In-Depth Look
03/05/2020 Abstracting and Coding Boot Camp
07/09/2020 Navigating the 2020 Survey Application Record (SAR)
03/04/2021 Abstracting and Coding Boot Camp 2021
02/03/2022 Data Item Relationships 2022
03/03/2022 Abstracting and Coding Boot Camp 2022
04/14/2022 Hematopoietic and Lymphocytic Neoplasms 2022
06/02/2022 Central Nervous System 2022
07/07/2022 Back to the future: What Year is it and What Did I Miss?
08/04/2022 Solid Tumor Rules 2022
09/01/2022 Coding Pitfalls 2022
02/02/2023 Data Item Relationships 2023
05/04/2023 Lower GI Part 1 2023
06/01/2023 Lower GI Part 2 2023
07/13/2023 IT Worked for Me: In "FUN" matics in the Cancer Registry
09/07/2023 Coding Pitfalls 2023
12/14/2023 Radiology and Radiation (RR) 2023
01/10/2024 Liver and Bile Ducts 2024
09/05/2024 Coding Pitfalls 2024
10/03/2024 Larynx and Base of Tongue 2024
Larynx and Base of Tongue Case Review
12/05/2024 Hematopoietic Part 1 2024
01/08/2025 Hematopoietic Part 2 2025
07/10/2025 Leveraging Technology to Improve Efficiency in Hospital Based Cancer Registries
Incidence Data
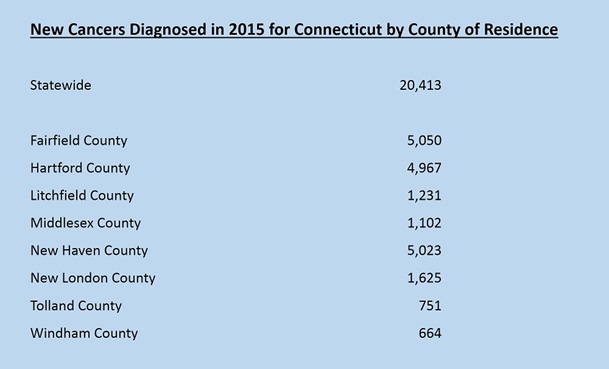
*Click here for cancer site-specific statistics by County of Residence.
Useful links for registrars:
 |
National Cancer Institute |  |
|
 |
 |
Tumor Registrars Association of Connecticut | |
 |
National Cancer Registrars Association |
For more information, please contact CTR at (860) 509-7163.
This work has been supported by federal funds from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN261201800002I.

