Frequently Asked Questions: THC-infused, hemp, or hemp derived beverages
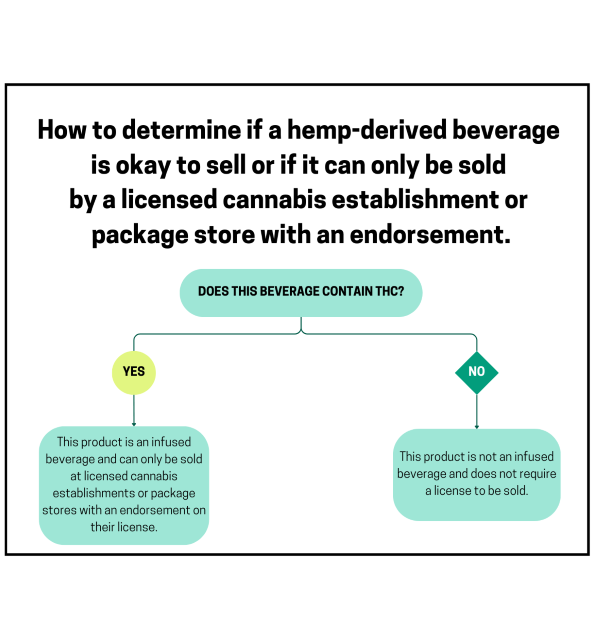
Can I sell THC-infused, hemp, or hemp derived beverages at my store?
THC-infused, hemp, or hemp-derived beverages are only permitted for sale in Connecticut in cannabis retail establishments (dispensary facilities, hybrid retailers, retailers) and package stores with an endorsement to sell THC-infused beverages. Infused beverages can contain no more than 3 mg of THC per container, and each container must be at least 12 ounces.
Examples
- Allowed: A standard 12 ounce can of seltzer containing 3 mg of THC per can.
- Not Allowed: A standard 12 ounce can of seltzer containing 10 mg and two 5 mg servings. A standard 12 ounce can of seltzer containing one 5 mg serving.
Can I sell beverage products that use the words cannabis, cannabis-infused, or cannabis-derived?
No, the law prohibits products that imply that they are cannabis.
Examples:
- Allowed: “THC-infused,” “Hemp-infused,” or “Hemp-derived.”
- Not Allowed: “Cannabis-infused,” “Cannabis Seltzer,” “Cannabis-water” or “Canna-beverage.”
Do beverages have specific labeling requirements?
Yes, each individual beverage container must be packaged or labeled with the following information:
- A scannable barcode, website address or quick response code that is linked to the beverage laboratory testing results performed by an independent testing laboratory, which lists all the following information:
- The name of the product
- The name, address and telephone number of such product's manufacturer, packer and distributor, as applicable
- The batch number associated with the laboratory testing results of the beverage in final form
- The concentration of cannabinoids present in the product, including, but not limited to, total THC and any cannabinoids or active ingredients comprising at least 1 percent of the product
- The expiration or best by date for the beverage, if applicable.
- A clear and conspicuous statement disclosing that:
- Children, or those who are pregnant or breastfeeding, should avoid using the product prior to consulting with a health care professional concerning such product's safety.
- Products containing cannabinoids should be kept out of reach of children.
- The federal Food and Drug Administration has not evaluated the product for safety or efficacy.

